Adcirca (tadalafil) for pulmonary arterial hypertension
What is Adcirca for pulmonary arterial hypertension?
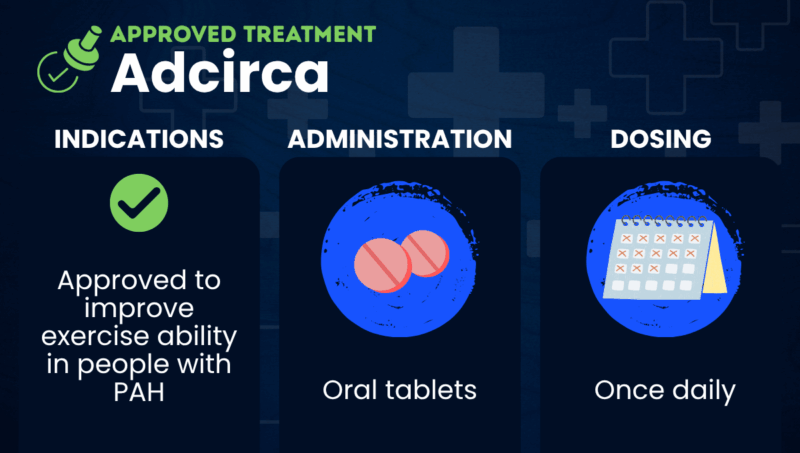
Adcirca (tadalafil) is a once-daily oral therapy approved in the U.S. to improve exercise ability in people with pulmonary arterial hypertension (PAH).
In PAH, the pulmonary arteries, which carry blood from the heart to the lungs, become narrowed, causing blood pressure to rise. This reduces blood flow and lowers the amount of oxygen circulating in the body, making physical activity more difficult.
Adcirca works by blocking an enzyme called phosphodiesterase type 5 (PDE5). As a result, there is an increase in a molecule called cyclic guanosine monophosphate that causes blood vessels to relax and widen. This relieves the abnormally high blood pressure in the pulmonary arteries, easing PAH symptoms.
Taken as tablets, Adcirca is marketed by United Therapeutics in the U.S. and by Eli Lilly in Europe. Generic versions are also available.
Adcirca’s active ingredient, tadalafil, is marketed under the brand name Cialis to treat erectile dysfunction and benign prostatic hyperplasia. Tadalafil is also one component of Opsynvi, a fixed-dose oral combination therapy that’s approved for PAH. An oral suspension formulation of tadalafil sold under the brand name Tadliq is also approved to improve exercise ability in people with PAH in the U.S.
Therapy snapshot
| Brand name | Adcirca |
| Chemical name | Tadalafil |
| Usage | Used to improve exercise ability in people with PAH |
| Administration | Oral tablets |
Who can take Adcirca?
Adcirca is approved in the U.S. for improving exercise ability in people with PAH (World Health Organization Group 1). U.S. regulators have not established the safety and effectiveness of Adcirca in children.
The therapy is also widely approved for treating PAH in other countries, although specific indications vary. In Europe, it can be used for PAH patients, ages 2 and older, with slight or marked limitations of physical activity (WHO Functional Class II or III).
Adcirca is contraindicated, or not recommended for use:
- in combination with organic nitrates or guanylate cyclase stimulators — the PAH therapy Adempas, for example — which could result in sudden or unsafe drops in blood pressure
- in patients with a history of hypersensitivity, or severe allergic reaction, to Adcirca or Cialis
How is Adcirca administered?
Adcirca is available as film-coated oral tablets to be taken by mouth, with or without food. The recommended dose is 40 mg once daily (two 20 mg tablets).
Dose adjustments may be needed in patients with kidney or liver function impairments, or in those taking the antiretroviral medication ritonavir.
Adcirca in clinical trials
Adcirca’s approval for PAH in the U.S. was based on data from PHIRST-1 (NCT00125918), a Phase 3 clinical trial that involved adults and adolescents with PAH who received Adcirca at one of four daily doses, or a placebo, for 16 weeks (about four months). Data showed that:
- Adcirca significantly improved exercise capacity when used at its approved daily dose (40 mg). After 16 weeks, the distance patients could walk in six minutes had increased by a mean of 33 meters, or about 108 feet, with the Adcirca dose relative to the placebo.
- Adcirca also delayed and reduced overall rates of clinical worsening, while improving health-related quality of life compared with the placebo.
PHIRST-1 participants could then receive Adcirca for up to another year in an open-label extension study called PHIRST-2 (NCT00549302). Adcirca continued to be well-tolerated and led to sustained clinical benefits over the extension period.
Adcirca side effects
The most commonly reported side effect of Adcirca in clinical trials was headache.
Adcirca may also cause less common, but serious side effects, including:
- hypotension (low blood pressure) that may be symptomatic, especially in people with underlying cardiovascular conditions
- sudden vision loss
- sudden decrease or loss of hearing
- prolonged and painful erections requiring urgent care
If fluid buildup in the lungs occurs with Adcirca, a person should be evaluated for pulmonary veno-occlusive disease, a rare cause of PAH. Adcirca is not recommended for people with this condition.
Adcirca may interact with certain medications, including other phosphodiesterase type 5 (PDE5) inhibitors. Patients should tell their healthcare providers about all medications they are using.
Pulmonary Hypertension News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 


