Adempas (riociguat) for pulmonary hypertension
What is Adempas for pulmonary hypertension?
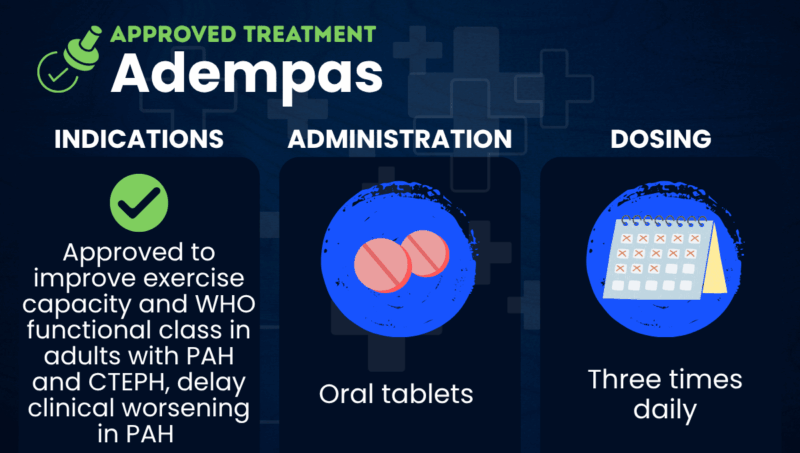
Adempas (riociguat) is an oral therapy approved to improve exercise capacity and World Health Organization (WHO) functional class in adults with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). It’s also indicated for delaying clinical worsening in PAH.
PAH and CTEPH are types of pulmonary hypertension (PH), a group of conditions characterized by high pressure in the blood vessels, called the pulmonary arteries, that supply blood to the lungs. In PAH, this is caused by the narrowing of the pulmonary arteries, while in CTEPH, it occurs because clots form in these vessels. Both conditions lead to symptoms such as shortness of breath and fatigue that can make physical activity difficult.
Adempas is a soluble guanylate cyclase (sGC) stimulator, which works to enhance a molecular pathway that promotes vasodilation, or the widening of blood vessels to increase blood flow. In this pathway, the natural chemical nitric oxide activates the sGC protein, which in turn boosts production of the vasodilation-triggering compound cyclic guanosine monophosphate (cGMP).
People with PH often have low nitric oxide levels, limiting natural activation of this pathway. Taken as oral tablets, Adempas works to overcome this by making sGC more sensitive to nitric oxide while also independently binding to and activating sGC. This indirectly boosts cGMP production, promoting blood flow through the pulmonary arteries to ease symptoms.
Bayer developed Adempas and markets it in the U.S. The company collaborates with Merck to commercialize it in some other countries. A generic version is available.
Therapy snapshot
| Brand name | Adempas |
| Chemical name | Riociguat |
| Usage | Used to improve exercise capacity and WHO functional class in adults with PAH and CTEPH, and to delay clinical worsening in PAH |
| Administration | Oral tablets |
Who can take Adempas?
Adempas is approved in the U.S. to:
- improve exercise capacity and WHO functional class in adults with persistent or recurrent CTEPH (WHO Group 4) after surgical intervention or in those who have an inoperable form of the disease
- increase exercise capacity and improve WHO functional class, as well as delay clinical worsening, in adults with PAH (WHO Group 1)
People who are pregnant should not use Adempas, which carries a boxed warning that it could cause fetal harm. For this reason, Adempas is only available to female patients through a restricted access program. Adempas is also contraindicated for, or not recommended for use by, patients who:
- are using other sGC stimulators, nitrates, nitric oxide donors in any form, or phosphodiesterase inhibitors such as the approved PAH therapies Adcirca (tadalafil) and Revatio (sildenafil)
- have PH associated with idiopathic interstitial pneumonia
Although not strictly contraindicated, Adempas is also not recommended for people with severe kidney or liver impairments, or those with pulmonary veno-occlusive disease (PVOD), a rare cause of PAH.
Adempas is approved in the European Union for adults with CTEPH, as well as adults and children, ages 6 and older, with PAH.
How is Adempas administered?
Adempas comes in film-coated oral tablets. The initial recommended dose is 1 mg, taken three times daily, which is slowly increased as tolerated up to a maximum of 2.5 mg, three times daily.
The starting dose may be lower for individuals who may not tolerate the blood pressure-lowering effects of Adempas or who are taking certain medications. Dose adjustments may also be needed for people who smoke or who stop smoking.
Individuals with difficulty swallowing pills can crush the medication and mix it with water or soft foods immediately before administration.
Adempas in clinical trials
The U.S. approval of Adempas for adults with CTEPH and PAH was supported by data from two Phase 3 clinical trials.
- CHEST-1 (NCT00855465) demonstrated that about four months of treatment with Adempas in people with CTEPH safely lowered resistance to blood flow through the pulmonary arteries compared with a placebo. Treated participants also showed improvements in exercise capacity and WHO functional class relative to those on the placebo.
- PATENT-1 (NCT00810693) showed similar findings in individuals with PAH over a treatment period of about three months. Compared with a placebo group, the Adempas group saw significant clinical benefits, such as increases in exercise capacity, lower resistance to blood flow in the pulmonary arteries, improved WHO functional class, and longer time to clinical worsening.
Long-term extension studies dubbed CHEST-2 (NCT00910429) and PATENT-2 (NCT00863681), which involved participants from the clinical trials, continued testing Adempas in these patient populations. Their results generally supported safe and effective long-term use of the medication.
An ongoing Phase 3 trial called PATENT-CHILD (NCT02562235) is testing Adempas in children with PAH, ages 6 to 17. Its initial findings suggested an acceptable safety profile and showed evidence of increased exercise capacity.
Adempas side effects
The most common side effects of Adempas for PAH and CTEPH include:
- headache
- stomach inflammation/indigestion
- dizziness
- nausea
- diarrhea
- low blood pressure (hypotension)
- vomiting
- low red blood cell count (anemia)
- acid reflux
- constipation
The boxed warning for Adempas indicates that the medication should not be administered to a pregnant female because it may cause fetal harm.
Female patients of reproductive potential should be advised of these risks, and must exclude pregnancy before starting treatment. Pregnancy also must be excluded monthly during treatment and for one month after stopping treatment. Effective forms of contraception must be used during treatment and for one month after the last dose.
Adempas also comes with warnings for uncommon, but potentially serious, side effects, including:
- symptomatic hypotension in people with certain medical conditions or who are on certain medications
- bleeding
- worsening of PVOD
If fluid accumulates in the lungs during Adempas treatment, it could be a sign of PVOD. If confirmed, Adempas should be stopped.
Pulmonary Hypertension News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 


