Lightweight, Portable Devices To Deliver Pulmonary-Blood-Vessel-Relaxing Gas For PAH Patients
Written by |
Inhaled nitric oxide (NO) is a proven life-saving therapy for newborns, children and adults with several dangerous conditions, including Pulmonary Arterial Hypertension (PAH) and Chronic Obstructive Pulmonary Disease (COPD). However the treatment availability to patients has been limited due to the size, weight, and complexity of equipment needed to administer the gas and the procedure’s accordingly high price.
Those limitations could soon be addressed by a technique developed by a research team led by the Massachusetts General Hospital (MGH) physician who pioneered the therapeutic use of inhaled nitric oxide. The MGH researchers have developed a lightweight, portable system that produces NO (which should not be confused with the anesthetic gas nitrous oxide AKA “laughing gas”) from the air by means of an electrical spark catalyst.
Production of the mono-nitrogen oxides NO and nitrogen dioxide (NO2) via reaction of nitrogen and oxygen gases at high temperatures is well-established. They are produced naturally by lightning strikes for example, and by the operation of air-breathing internal combustion engines, creating a substantial proportion of air polluting emissions (NO2 is toxic) from motor vehicles. Indeed NOx gases are formed whenever nitrogen — the largest component of air which contains (dry) 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.039% carbon dioxide, and small amounts of other gases — is exposed to high temperatures in the presence of oxygen.
However, the quest of the MGH scientists and their research colleagues was to develop a compact, efficient, and economical means of harnessing this natural process to produce therapeutic nitric oxide, leading to invention of a device they describe in an article published in the July 1 issue of the journal Science Translational Medicine, entitled Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy” (Science Translational Medicine 01 Jul 2015: Vol. 7, Issue 294, pp. 294ra107 DOI: 10.1126/scitranslmed.aaa3097).
 The Science Translational Medicine paper is coauthored by Binglan Yu, PhD corresponding author, with Stefan Muenster, MD, Aron H. Blaesi, PhD, Donald B. Bloch, MD, and, and Warren M. Zapol, MD of the Anesthesia Center for Critical Care Research, Department of Anesthesia, Critical Care and Pain Medicine. Massachusetts General Hospital, Harvard Medical School in Boston. Dr. Bloch is also affiliated with the Massachusetts General Hospital, Harvard Medical School Division of Rheumatology, Allergy and Immunology.
The Science Translational Medicine paper is coauthored by Binglan Yu, PhD corresponding author, with Stefan Muenster, MD, Aron H. Blaesi, PhD, Donald B. Bloch, MD, and, and Warren M. Zapol, MD of the Anesthesia Center for Critical Care Research, Department of Anesthesia, Critical Care and Pain Medicine. Massachusetts General Hospital, Harvard Medical School in Boston. Dr. Bloch is also affiliated with the Massachusetts General Hospital, Harvard Medical School Division of Rheumatology, Allergy and Immunology.
The coauthors note that inhalation of nitric oxide (NO) produces selective pulmonary vasodilation and is therefore an effective therapy for treating pulmonary hypertension in adults and children, but that currently the United States, the average cost for five days of inhaled NO for persistent pulmonary hypertension in newborns is about $14,000 — the high cost attributable to conventional NO therapy’s reliance on gas cylinders and distribution, a complex delivery device, gas monitoring and calibration equipment, and a trained respiratory therapy staff.
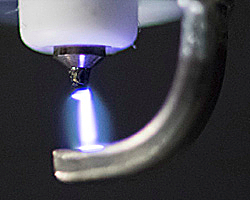
The investigators say the objective of their study was to develop a lightweight, portable device to serve as a simple and economical method of producing pure NO from air for bedside or portable use. In the course of their research, two prototype NO generators were designed and tested: one an offline NO generator and the other an inline NO generator spliced directly into the inspiratory line in a way that synchronizes generation of NO during inhalation with pulsed delivery of oxygen and other gases to be inhaled, reducing the NO that would be lost during exhalation. Both generators use pulsed electrical discharges to produce therapeutic grade NO (at concentrations of 5 to 80 parts per million) at gas flow rates of 0.5 to 5 liters/min. NO was produced from atmospheric air, as well as from gas mixtures containing up to 90% O2 and 10% N2.
Potentially toxic gases produced in the plasma, including nitrogen dioxide (NO2) and ozone (O3), were removed using a calcium hydroxide scavenger. The scientists found that an iridium spark electrode produced the lowest ratio of NO2/NO. In experiments using lambs with acute pulmonary hypertension, breathing electrically generated NO produced pulmonary vasodilation and reduced pulmonary arterial pressure and pulmonary vascular resistance index. The investigators conclude that electrical plasma NO generation produces therapeutic levels of NO from air, and that after scavenging to remove NO2 and O3 and filtration to remove particles, electrically produced NO can provide safe and effective treatment of pulmonary hypertension.
 “Nitric oxide is used to treat about 35,000 hospitalized U.S. patients each year mostly adults with pulmonary hypertension and infants with a condition called persistent pulmonary hypertension of the newborn (PPHN),” says Warren M. Zapol, MD, director of the MGH Anesthesia Center for Critical Care Research and emeritus chief of Anesthesia and Critical Care at the hospital, and senior author of the Science Translational Medicine report. “But NO therapy is very expensive here at MGH five days treatment of a newborn with PPHN costs approximately $14,000, and current systems use gas delivered in heavy tanks, making ambulatory treatment impractical. Our new system can economically make NO from the nitrogen and oxygen in the air using only small amounts of electric power. This device could enable trials of NO to treat patients with chronic lung diseases and certain kinds of heart failure and would make NO therapy available in parts of the world that dont have the resources that are currently required.”
“Nitric oxide is used to treat about 35,000 hospitalized U.S. patients each year mostly adults with pulmonary hypertension and infants with a condition called persistent pulmonary hypertension of the newborn (PPHN),” says Warren M. Zapol, MD, director of the MGH Anesthesia Center for Critical Care Research and emeritus chief of Anesthesia and Critical Care at the hospital, and senior author of the Science Translational Medicine report. “But NO therapy is very expensive here at MGH five days treatment of a newborn with PPHN costs approximately $14,000, and current systems use gas delivered in heavy tanks, making ambulatory treatment impractical. Our new system can economically make NO from the nitrogen and oxygen in the air using only small amounts of electric power. This device could enable trials of NO to treat patients with chronic lung diseases and certain kinds of heart failure and would make NO therapy available in parts of the world that dont have the resources that are currently required.”
An MGH release notes that nitric oxide was long considered to be only a toxic pollutant gas, but that in the mid-1980s, three U.S. investigators discovered its utility as a signal-transmitting molecule naturally used by the pulmonary, cardiac and other systems — a discovery that received the 1998 Nobel Prize. Among its many functions, NO relaxes muscles surrounding blood vessels, which reduces blood pressure a property that led Dr. Zapol and his colleagues to investigate NO’s use in treatment of hypertension in the blood vessels supplying the lungs. The MGH researchers’ discovery that inhaled NO selectively relaxed pulmonary vessels without producing a systemic drop in blood pressure led to the therapy’s FDA approval in 1999 for \ treatment of PPHN and other lung diseases in newborns. In 2003 Dr. Zapol and his former research fellow Claes Frostell, MD, PhD, received the Inventor of the Year award from the Intellectual Property Owners Association for their development of a system to safely deliver inhaled NO.
Since inhaled NO therapy received FDA approval, the gas has been supplied to hospitals in large compression tanks and administered to patients through bulky and complex delivery devices operated by trained respiratory therapists. However, in 1992 Dr. Zapol and the MGH were issued a patent for a system his research team had developed to produce NO by means of electrical spark. Although the discovery was licensed by two medical and industrial gas companies, the technology was never developed, possibly because medical use of inhaled NO was only beginning to be accepted at the time.
Additionally, the system originally invented by the MGH team was too large for use in outpatient settings. Despite these limitations, increased therapeutic use of NO along with technological advances, including the availability of miniaturized electronic circuitry, allowed the MGH scientists to develop the system described in the journal report.
Since generation of NO by an electric spark can also produce the toxic gases nitrogen dioxide and ozone, along with metal fragments from the electrode, both systems use calcium hydroxide and air filters to absorb and remove those byproducts.
A series of experiments conducted by the research team helped them determine the best metal to use for the electrode, which was determined to be iridium, and the optimal timing and number of electric sparks. Animal model testing revealed that electrically generated NO produced by both of the prototype systems described above was as effective as tank-delivered gas in relieving pulmonary hypertension. They also discovered that while a gas mixture containing 50 percent oxygen produced the highest NO levels, the amount produced from ambient air was sufficient for therapeutic use. Both prototype systems continued to deliver therapeutic levels of NO for up to 10 days, and subsequent experiments with the inline system not included in the referenced Science Translational Medicine paper have continued for up to 28 days. “There’s no reason to think the system could not stably produce NO for even longer periods of time,” notes Dr. Zapol, who is also the Reginald Jenney Professor of Anaesthesia at Harvard Medical School.

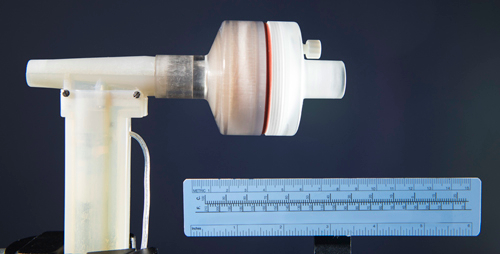
Photo caption: In this nitric oxide (NO) generator, which can be incorporated into a mechanical ventilator or anesthesia machine, air is taken in through the inlet on the left and passes by the electrode at the top of the vertical segment, where a series of sparks generates NO gas. The cylindrical chamber contains calcium hydroxide and a filter to remove toxic byproducts before the NO-enriched air exits on the right to be inhaled by the patient. (Photo credit – Brian Wilson, MGH Photography Department)
“It’s amazing how long this system continues to make NO, Dr. Zapol continues. “Once we’ve shown that this can safely be used in human patients with pulmonary hypertension and we’ve got a clinical trial in progress right now well be able to conduct studies of inhaled NO delivered in ambulatory settings, including patients’ homes, to treat chronic pulmonary hypertension, right-sided heart failure and chronic obstructive pulmonary disease.”
 Richard Channick, MD, director of the MGH Pulmonary Hypertension Program, says, “This advance has great potential for our patients. If proven safe and effective, electrically generated NO therapy will greatly enhance our ability to treat many forms of pulmonary hypertension.”
Richard Channick, MD, director of the MGH Pulmonary Hypertension Program, says, “This advance has great potential for our patients. If proven safe and effective, electrically generated NO therapy will greatly enhance our ability to treat many forms of pulmonary hypertension.”
 Marc Semigran, MD, medical director of the MGH Heart Failure and Cardiac Transplant Service observes: “The ability to safely administer inhaled NO chronically to heart failure patients could improve the lives of many of the millions of patients with secondary pulmonary hypertension.” Both physicians are collaborators with Dr. Zapol’s research team, although they are not co-authors of the current study.
Marc Semigran, MD, medical director of the MGH Heart Failure and Cardiac Transplant Service observes: “The ability to safely administer inhaled NO chronically to heart failure patients could improve the lives of many of the millions of patients with secondary pulmonary hypertension.” Both physicians are collaborators with Dr. Zapol’s research team, although they are not co-authors of the current study.
Several additional patent applications covering the new system have been filed, and an option to license those patents has been granted to the startup company Third Pole, Inc.
Sources:
Massachusetts General Hospital
Wikipedia
Photo Credits:
Massachusetts General Hospital