INOmax Vasodilator Device For Pulmonary Hypertension Approved In Australia And Japan
Dublin, Ireland, based biopharmaceutical company Mallinckrodt plc reports that its INOmax (nitric oxide) vasodilator inhalation device has received regulatory approval in Australia and Japan for treating pulmonary hypertension in conjunction with heart surgery.
The Australian Therapeutic Goods Administration (TGA) has approved INOmax for peri- and post-operative pulmonary hypertension in conjunction with cardiovascular surgery in neonates through adolescents up to age 17. INOmax was previously approved in 2007 for the treatment of hypoxic respiratory failure (HRF) associated with pulmonary hypertension, a potentially life-threatening condition in newborns.
Japan’s Ministry of Health, Labour and Welfare (MHLW) has also approved INOmax, marketed there as INOflo/INOvent, for peri-operative pulmonary hypertension in conjunction with cardiovascular surgery for use in neonates through adults.
In newborns with HRF associated with concurrent pulmonary hypertension, Mallinckrodt says INOmax has been shown to improve oxygenation and reduce the need for a highly invasive surgical procedure known as extracorporeal membrane oxygenation (ECMO). The new indications in Japan and Australia expand INOmax’s use to broader patient populations.
The company notes that INOmax was the first pharmaceutical gas approved in both Australia and Japan in 2007 and 2008, respectively, for treatment of hypoxic respiratory failure (HRF), a potentially life-threatening condition in newborns.
The vasodilator is approved in the United States to improve oxygenation and reduce need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents. However, INOmax is not approved by the FDA for use in patients older than neonates or for use in surgical settings.
Mallinckrodt reports that clinical studies have also demonstrated INOmax’s efficacy in treating hypoxic respiratory failure (HRF) associated with pulmonary hypertension (PH).
 INOmax is a vasodilator, which, in conjunction with ventilatory support and other appropriate agents, is indicated for the treatment of term and near-term (>34 weeks) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension, where it improves oxygenation and reduces the need for extracorporeal membrane oxygenation.
INOmax is a vasodilator, which, in conjunction with ventilatory support and other appropriate agents, is indicated for the treatment of term and near-term (>34 weeks) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension, where it improves oxygenation and reduces the need for extracorporeal membrane oxygenation.
When inhaled, nitric oxide selectively dilates the pulmonary vasculature, and because of efficient scavenging by hemoglobin, has minimal effect on the systemic vasculature. INOmax causes vasodilation in the pulmonary vasculature by increasing cGMP levels through the activation of guanylate cyclase.
Inhalation of INOmax offers rapid inactivation, with clinical responses seen in as little as 30 minutes, and its short half-life minimizes systemic effects. Rapid metabolism in the blood converts nitric oxide to metabolites that are predominantly cleared by the kidneys. However, methemoglobinemia (a blood disorder in which an abnormal amount of methemoglobin — a form of hemoglobin — is produced) is a dose-dependent side effect of inhaled nitric oxide therapy, so therefore, methemoglobin levels should be monitored during INOmax administration.
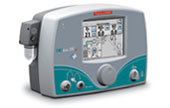
INOmax is dedicated to developing next-generation technologies, and INOmax delivery systems offer a range of innovative features.
• Continuous innovation supports evolving information and technology needs
• Compatible with more than 63 ventilation systems, including HFOV and noninvasive modalities
• Allows for operator-determined concentrations of nitric oxide (NO) in the breathing unit
• Provides for a nitric oxide concentration that is constant throughout the respiratory cycle
• Monitors for NO, oxygen (FiO2), and nitrogen dioxide (NO2)
• Prevents generation of excessive inhaled NO2
• Meets all FDA-required specifications
“The approval of INOmax in Australia and Japan for cardiovascular surgery represents a significant milestone for Mallinckrodt and INOmax,” said Tom Englese, General Manager, Mallinckrodt. “Worldwide, there is a tremendous need for therapies for patients undergoing cardiac surgery. We look forward to helping meet these needs while broadening our reach in global markets.”
For more information, visit:
https://www.mallinckrodt.com
and
https://inomax.com/
Sources:
Mallinckrodt plc
NiH MedlinePlus Medical Encyclopedia







