Actelion’s Selexipag Reduces PAH Patient Morbidity/Mortality in Phase 3 Clinical Trial
 Actelion Ltd. met the primary endpoint of its pivotal Phase 3 clinical trial of selexipag in pulmonary arterial hypertension (PAH) patients. Morbidity and mortality were decreased in patients dosed with selexipag relative to placebo.
Actelion Ltd. met the primary endpoint of its pivotal Phase 3 clinical trial of selexipag in pulmonary arterial hypertension (PAH) patients. Morbidity and mortality were decreased in patients dosed with selexipag relative to placebo.
“I am overwhelmed by the result of this long-term outcome study that evaluated selexipag in a setting where 80 percent of patients already received oral PAH therapy at baseline,” said Jean-Paul Clozel, MD and Chief Executive Officer of Actelion, in a news release. “Together with our partner Nippon Shinyaku, we are now one step closer to bringing an effective oral therapy targeting the prostacyclin pathway to the PAH community. We will now work diligently to complete the analyses with the goal to initiate first regulatory filings with Health Authorities as soon as possible.”
[adrotate group=”4″]
Morbidity and mortality were reduced by 39% in patients receiving selexipag compared to placebo. Adverse effects were common to those seen in patients taking other prostacyclin therapies (such as headache, diarrhea, nausea, jaw pain, and vomiting) and occurred in more selexipag-treated patients than placebo-treated patients. Fourteen percent of selexipag patients withdrew from treatment due to adverse events, compared to seven percent of placebo patients.
“The Prostacyclin (PGI2) Receptor Agonist In Pulmonary Arterial Hypertension (GRIPHON) study results hold the promise that selexipag might open up the prostacyclin pathway to different groups of patients given the consistent efficacy findings across key subgroups evaluated in this long-term outcome study,” commented Vallerie McLaughlin, MD, Director of the Pulmonary Hypertension Program in the Division of Cardiovascular Medicine at University of Michigan. “In addition, GRIPHON has shown once again that registration studies that follow the robust morbidity/mortality definitions–as recommended by the 4th and 5th World symposia on Pulmonary Hypertension–have the potential to deliver truly meaningful clinical information.”
[adrotate group=”3″]
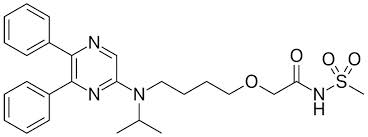
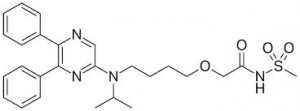 Selexipag is the first selective oral prostacyclin IP receptor agonist. It was studied in 1,156 patients during the GRIPHON study. Dosing was initiated at 200 micrograms and increased by 200 micrograms twice daily up to a maximum of 1600 micrograms twice daily. Treatment was continued for up to 4.3 years. Long-term efficacy and safety were of interest and were compared between oral selexipag and placebo.
Selexipag is the first selective oral prostacyclin IP receptor agonist. It was studied in 1,156 patients during the GRIPHON study. Dosing was initiated at 200 micrograms and increased by 200 micrograms twice daily up to a maximum of 1600 micrograms twice daily. Treatment was continued for up to 4.3 years. Long-term efficacy and safety were of interest and were compared between oral selexipag and placebo.
“I have been prescribing intravenous prostacyclin therapies in PAH patients for almost twenty years,” said Gérald Simonneau, MD, Professor of Pulmonology and Head of the Department of Pulmonary Disease and Intensive Care Unit at Université Paris-Sud, Le Kremlin-Bicêtre, France. “Today’s GRIPHON results represent a major step forward. For the first time, with selexipag, we have an oral compound acting on the prostacyclin pathway showing a significant risk reduction on a highly clinically relevant endpoint.”
More study results will be announced at upcoming congresses and published in peer-reviewed journals.







