Riociguat Provides Hope for Difficult-to-Treat PAH Patients
 Some of the most difficult-to-treat patients with pulmonary hypertension are those with chronic thromboembolic pulmonary hypertension (CTEPH). These patients are in the most severe category of pulmonary hypertension: World Health Organization (WHO) Group 4. CTEPH patients have curative potential if they have no resectable lesions or contraindications for surgery and have access to surgical centers. However, a majority of patients do not fit this description, and clinicians must resort to multidisciplinary approaches for treatment.
Some of the most difficult-to-treat patients with pulmonary hypertension are those with chronic thromboembolic pulmonary hypertension (CTEPH). These patients are in the most severe category of pulmonary hypertension: World Health Organization (WHO) Group 4. CTEPH patients have curative potential if they have no resectable lesions or contraindications for surgery and have access to surgical centers. However, a majority of patients do not fit this description, and clinicians must resort to multidisciplinary approaches for treatment.
Current medicinal options for pulmonary hypertension include prostanoids, endothelin-receptor antagonists (ERAs) such as Macitentan, phosphodiesterase type 5 inhibitors, and calcium channel blockers. A new addition to this list is riociguat, a novel drug developed by Bayer under the name Adempas. Riociguat was approved by the FDA in October 2013. According to a press release from Bayer, riociguat is indicated for use in “adults with persistent/recurrent CTEPH after surgical treatment or inoperable CTEPH to improve exercise capacity and WHO functional class.” It is also indicated to treat patients in the less severe WHO Group 1 category to improve exercise capacity and prevent WHO functional class worsening.
[adrotate group=”4″]
Two large Phase 3 clinical trials were conducted by Bayer, and the results were presented in October 2013 at CHEST 2013, the annual meeting of the American College of Chest Physicians. PATENT-2 and CHEST-2, which were long-term extension studies of PATENT-1 and CHEST-1, continued to study the efficacy and safety of riociguat treatment, with the primary outcome of a change in six-minute walking distance.
PATENT-1 had stellar results, with riociguat-treated patients improving their exercise capacity and hemodynamic parameters. An editorial overview from Mednet interviewed two Directors of the Pulmonary Hypertension Programs at two Canadian Hospitals following the Annual Congress of the European Respiratory Society. Dr. John Granton, from Toronto General Hospital, commented on the PATENT-1 trial, “Many centers have adopted a ‘treat to target’ philosophy of care for patients with pulmonary arterial hypertension. Although this is an exploratory analysis, this finding aligns with how we actually practice. Therefore [the trial] results may resonate with practicing clinicians.” Dr. John Swiston, of Vancouver General Hospital, confirmed the study “increase[s] my confidence in the efficacy of riociguat in patients with pulmonary arterial hypertension.”
[adrotate group=”3″]
Concerning CHEST-1, Dr. Swiston now “anticipate[s] the use of riociguat in these patients.” Improvements in exercise capacity at 16 weeks were significant to any seen in patients treated with a placebo. Beyond these 16 weeks, in CHEST-2, “These results support the notion that the initial treatment effect is likely robust and sustained,” stated Dr. Granton. “Longer-term data also supports that the agent is safe and provides a more reliable side effect profile.” PATENT-2 results continued to bolster confidence in riociguat.
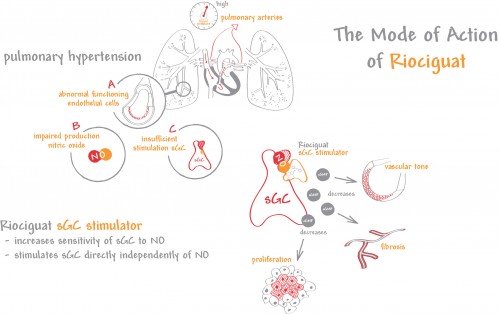
Riociguat is a guanylate cyclase (sGC) activator. The downstream effect of guanylate cyclase in the context of pulmonary hypertension is vasodilation. Riociguat can act independent from nitric oxide, but it can also work synergistically to prevent aggregations and proliferation in the vessels. It will be an important addition to the medicines prescribed by clinicians, such as Drs. Swiston and Granton, who deem approximately 40% and 10% of their CTEPH patients as inoperable.







