Aironite Mist Lowers Blood Pressure in Hearts of Pulmonary Hypertension Patients, Trial Update Shows
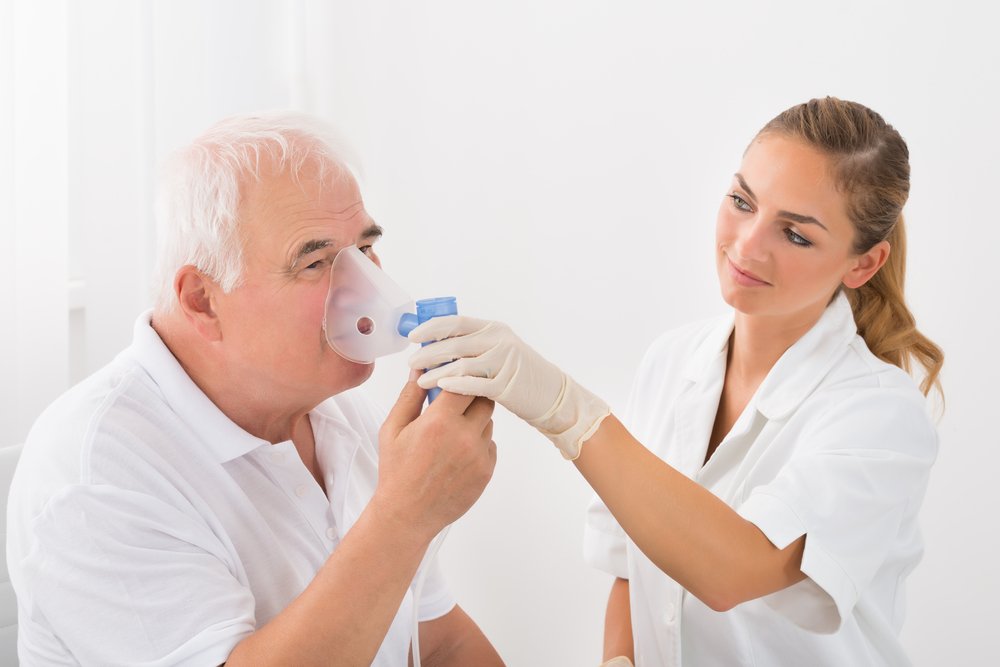
Savara’s inhaled sodium nitrite therapy Aironite lowered blood pressure in the upper and lower heart chambers and pulmonary artery of pulmonary hypertension patients, according to an update of a Phase 2 clinical trial.
The most improvement occurred in PH patients with a condition known as heart failure with preserved ejection fraction, researchers said. That is a form of heart failure in which the left ventricle is pumping blood at more than half its normal capacity.
Savara presented the results at the Drug Discovery And Development Symposium For Pulmonary Hypertension in Berlin, July 10 and 11.
The company enrolled 41 patients in the open-label Phase 2 clinical trial (NCT01431313), according to Dr. Marc A. Simon, the trial’s lead investigator, who made the presentation. Simon is director of the University of Pittsburgh‘s Heart Failure Research and Clinical Hemodynamics Core Facility.
Aironite is a sodium nitrite solution that a nebulizer can change into a mist for easier access to the lungs. Nitrite widens blood vessels. It can also be turned into nitric oxide, which has vessel-dilating properties as well.
In the trial, researchers assessed the effectiveness of two doses of Aironite given about an hour apart. Ten heart failure patients with preserved ejection fraction participated in the study.
Aironite decreased the blood pressure in the heart’s right upper chamber, or atrium. It also reduced the pressure in the lower heart chambers and the pulmonary artery. And it lowered pulmonary capillary wedge pressure — an indirect measure of pressure in the upper left heart chamber.
In addition to heart failure patients, Aironite benefited patients with Group 3 PH — or pulmonary hypertension caused by other lung disorders.
Patients tolerated the treatment well, and researchers did not find any significant safety problems, Simon said.
The team published interim results of the trial in the Journal of Clinical Investigation Insights last year. The article reported that the trial had covered 36 patients. The updated results, involving five more patients, strengthen the case for the treatment.
“These additional results build upon prior interim data published last year in the Journal of Clinical Investigation,” Dr. Taneli Jouhikainen, Savara’s chief operating officer, said in a press release. The update demonstrates that Aironite “can significantly improve cardiopulmonary hemodynamics in HFpEF [pulmonary hypertension due to heart failure with preserved ejection fraction] as well as Group 3 PH patients, both clinical conditions which are inadequately treated by currently approved medicinal treatments,” he said.
“If the observed short-term improvements are translated into clinically meaningful functional improvements in our ongoing placebo-controlled studies in HFpEF patients, we believe the product will have exciting potential to be advanced towards Phase 3 studies and hopefully an eventual NDA [New Drug Application] filing” with the U.S. Federal Drug Administration, Jouhikainen added.
Savara is providing treatment and additional support in two Phase 2 studies (NCT02742129 and NCT02713126) that are looking at whether inhaled nitrite can improve exercise capacity in patients with PH and heart failure with preserved ejection fraction.
Mast Therapeutics developed Aironite as AIR001. Mast and Savara merged in April 2017, with Savara taking over the therapy’s development.







