Macitentan Significantly Reduces PAH-Related Hospitalization, Study Shows
 In a new report entitled “Effect of Macitentan on Hospitalizations: Results From the SERAPHIN Trial,” researchers describe the benefits of a Macitentan 10 mg dose in reducing the risk for hospitalization in patients with symptomatic pulmonary arterial hypertension. The report was published in the journal of the American College of Cardiac Foundation (JACC) Heart Failure.
In a new report entitled “Effect of Macitentan on Hospitalizations: Results From the SERAPHIN Trial,” researchers describe the benefits of a Macitentan 10 mg dose in reducing the risk for hospitalization in patients with symptomatic pulmonary arterial hypertension. The report was published in the journal of the American College of Cardiac Foundation (JACC) Heart Failure.
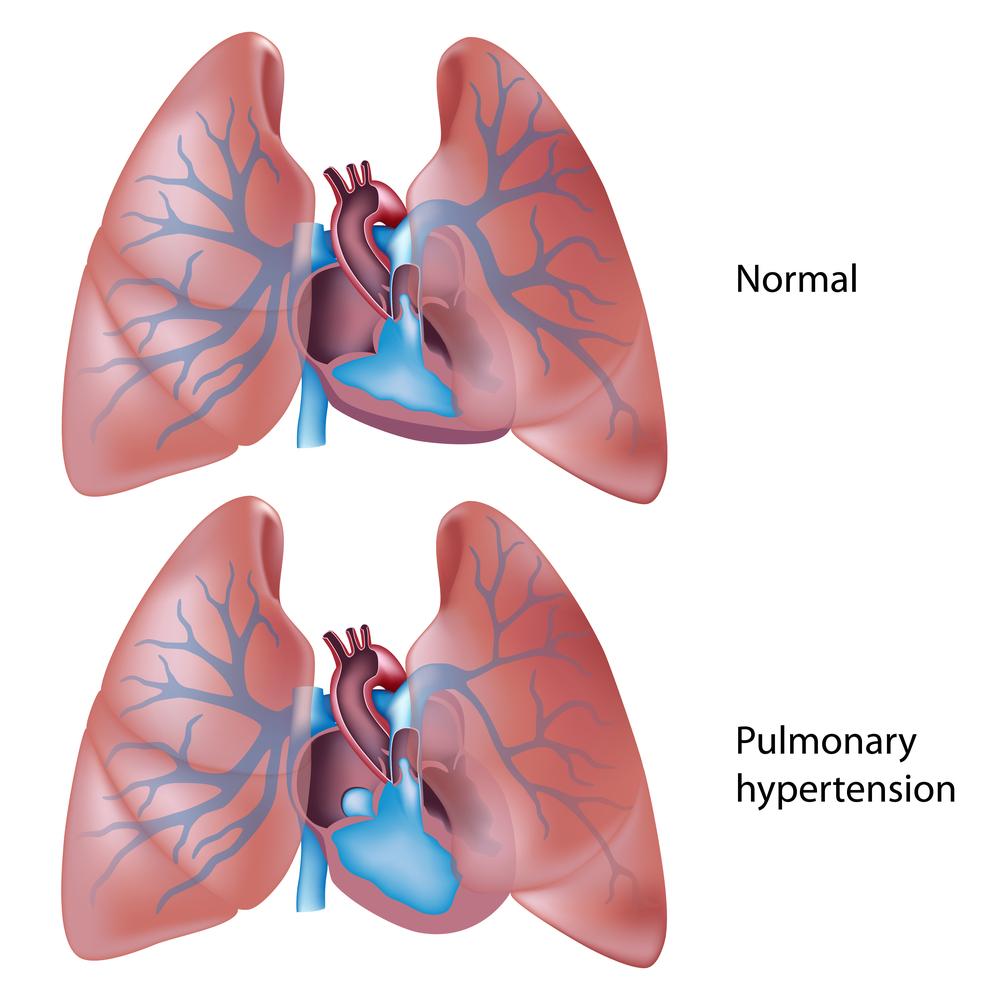
Pulmonary arterial hypertension (PAH) is a progressive, life-threatening disorder characterized by high blood pressure in the blood vessels (arteries) of the lungs due to arteries abnormal constriction. Patients with PAH often require hospitalization, which is known to decrease patients’ quality of life and increase the financial burden of this chronic disease.
[adrotate group=”4″]
In this new study, the authors present the results from a multi center (151 centers within 39 countries), double-blind, randomized phase III SERAPHIN (short for Study with an Endothelin Receptor Antagonist in Pulmonary arterial Hypertension to Improve clinical outcome) trial that enrolled 742 patients with symptomatic PAH. Patients were randomly assigned to receive placebo (250 patients), a dose of 3 mg of macitentan (250 patients) or a 10 mg of macitentan (242 patients). Macitentan is a newly developed dual endothelin receptor antagonist: these new class of drugs were shown to effectively combat several diseases, including PAH (here patients lungs produce excessive endothelin, protein that lead to blood vessels constriction increasing blood pressure), by blocking endothelin receptors and thus preventing the harmful effects of endothelin. The treatment with placebo and macitentan occurred over approximately two years.
[adrotate group=”3″]
Patients treated with either 3 mg or 10 mg of macitentan exhibited a reduction in the risk of all-cause hospitalization by 18.9% and 32.3%, respectively, when compared to the placebo control; the rate of hospitalizations (all-causes) together with days in the hospital were also decreased by 30.6% (3 mg macitentan) and 33.1% (10 mg macitentan), as well as the risk for PAH-related hospitalizations and numbers of hospital days, specifically by 42.7% and 51.6%, respectively.
The authors concluded the results from the phase III SERAPHIN trial show that 10 mg of macitentan significantly reduces risk, rate and days of hospitalizations in PAH patients, therefore highlighting the long-term benefits for PAH patients treated with macitentan.
In other developments in pulmonary hypertension, a study published in the journal Respiratory Medicine Case Reports revealed a case where a patient with idiopathic pulmonary fibrosis (IPF) was successfully treated for severe pulmonary hypertension (PH) with bosentan. The study is entitled “Bosentan for pulmonary hypertension secondary to idiopathic pulmonary fibrosis.”