FDA Extends Indication of VMS System Used For PAH To Include Heart Disease
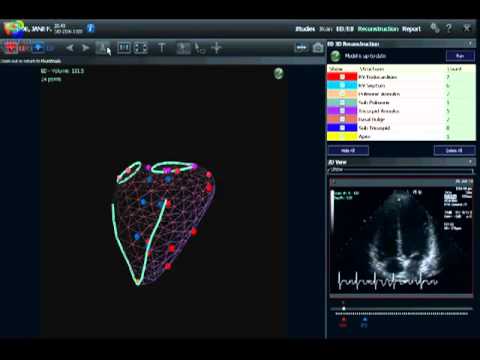
The U.S. Food and Drug Administration (FDA) has approved the extended use of a VMS Heart Analysis system developed and commercialized by the company VentriPoint Diagnosis, which is currently used in patients who suffer from pulmonary arterial hypertension (PAH). According to the new indication, the system may be used in diagnosing all kinds of heart disease to measure right heart function.
VMS is a medical device developed by VentriPoint to enable heart imaging and provides a more rapid and practical system in comparison with the use of MRI. The novel system captures images on the right side of the heart that can be used by physicians to evaluate patients’ heart function. Imaging the right side of the heart difficult due to its location, hidden by the ribs.
Therefore, being able to capture right heart images is a major advantage of any system, while VMS has been shown to be efficient in reducing analysis time from 10 to 30 minutes to only five. In addition, right heart function is among the most important indicators related to heart conditions. “The VMS system is indicated for use where RV (right ventricle) volumes and ejection fractions are warranted or desired,’’ explained the company in a statement.
“Physicians in the U.S. can now use the VMS on patients that they believe will benefit from assessment of RV function, without being limited to a specific condition,” said the vice-president for clinical affairs and development at VentriPoint, Jim Bodtke.
The system had already been approved by the FDA in March 2014 for the examination of the right ventricle in adult patients who suffer from PAH. With the extended indications approval, “the VMS can be used on the more than 20 million people that suffer from heart disease,” according to VentriPoint.
In addition, the system is also available in both Canada and Europe. Last July, the company released studies evaluating VMS, which was originally designed for congenital heart disease, with subsequent clearance for PAH and non-specific heart disease. A group of German investigators revealed the promising capacities of the technology to improve imaging, diagnosing, and treatment of patients with PAH and heart disease.







