Praxair’s Inhaled Nitric Oxide Therapy for Newborns with Pulmonary Hypertension Approved in Canada
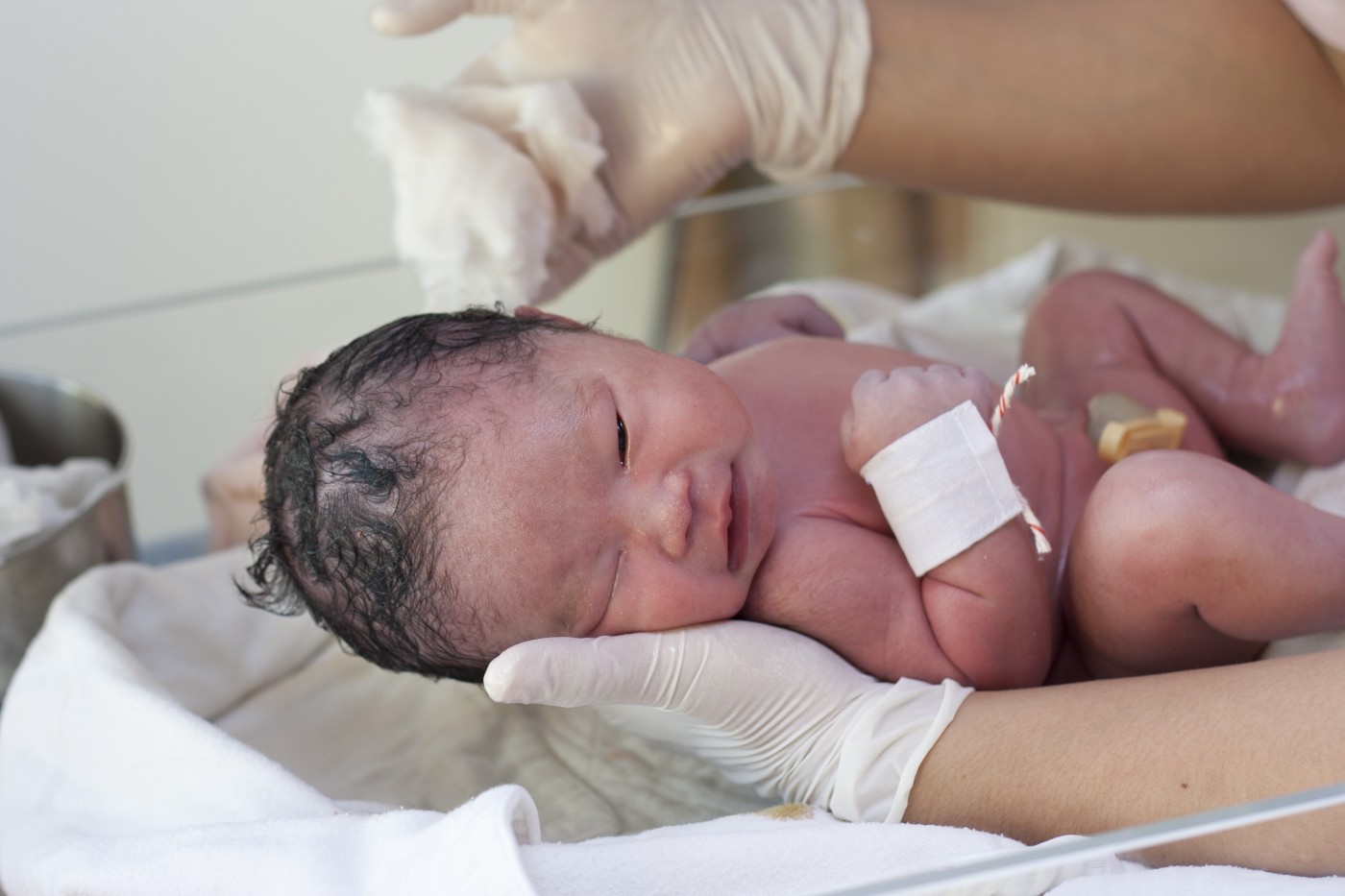
Praxair, Inc., announced that it has received approval, through a subsidiary, for the sale of its Noxivent brand of inhaled nitric oxide in Canada. Noxivent is indicated for the treatment of newborns, including some born prematurely, suffering respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension (PH).
PH in neonates can be especially difficult to manage and treat, occurring in a variety of clinical situations and exacerbated by hypoxia (lack of oxygen). A series of treatment options are available, and inhaled nitric oxide is the pulmonary vasodilator of choice.
Noxivent, the company’s brand of nitric oxide for inhalation, will be available through Praxair Canada in 800 ppm and 100 ppm concentrations. In conjugation with ventilator support and other agents, the treatment is appropriate for newborns and those born prematurely but at least 34 weeks into a pregnancy (late pre-term), diagnosed with hypoxic respiratory failure associated with PH.
Inhaled nitric oxide therapy, a selective pulmonary vasodilator that induces smooth muscle relaxation, has been shown to improve oxygenation and reduce the need for extra oxygen. Earlier this year, the company announced that it had acquired NOxBOX Ltd., a company that specializes in the manufacture of inhaled gas delivery and monitoring instruments for the medical market.
The NOxBOXi delivery device provides controlled delivery with precise and real-time monitoring of nitric oxide, nitrogen dioxide, and oxygen. This device is designed for in-hospital use and during transit and transfer situations, and can be used with invasive and non-invasive ventilation. Patient monitoring and management are important, as complications may arise due to toxic levels of nitrogen dioxide, and recurrence of PH due to weaning from inhaled nitric oxide.
“With this approval from Health Canada, we will pair the Noxivent product with the NOxBOXi™ delivery system, enabling us to provide our Canadian hospital customers with a comprehensive respiratory offering in a cost-effective manner,” Sean Durbin, president of Praxair Canada, said in a news release. “Our customers have been looking for choices in the marketplace and we are pleased to be able to deliver.”







