Tadliq (tadalafil) for pulmonary arterial hypertension
What is Tadliq for pulmonary arterial hypertension?
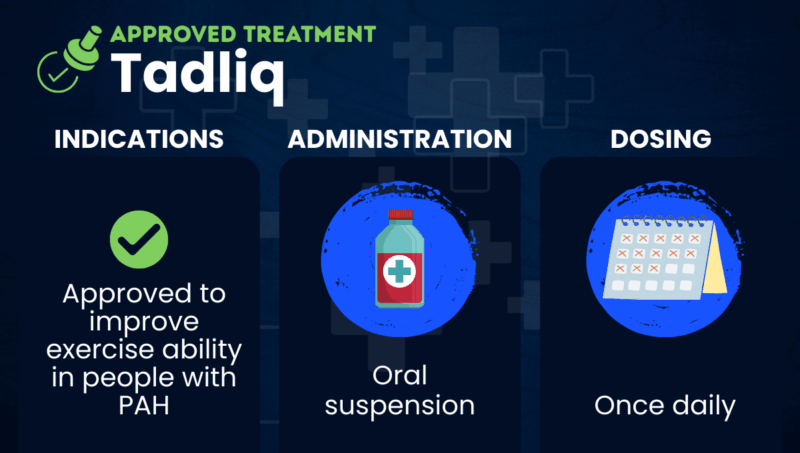
Tadliq is a liquid oral suspension formulation of tadalafil that’s approved in the U.S. to improve exercise ability in people with pulmonary arterial hypertension (PAH).
PAH occurs when the vessels that carry blood to the lungs become narrowed, impeding blood flow. As a result, blood pressure in the lungs rises, putting strain on the heart. Symptoms, including shortness of breath and excessive fatigue, can make it more difficult to be active.
Tadliq aims to reduce blood pressure in the lungs and improve blood flow by relaxing blood vessels (vasodilation). It works by inhibiting phosphodiesterase type 5 (PDE5), an enzyme that typically breaks down a vasodilation-promoting molecule called cyclic guanosine monophosphate.
CMP Pharma markets Tadliq, the only oral suspension formulation of tadalafil that’s approved by the U.S. Food and Drug Administration (FDA). Oral tablet versions of tadalafil, sold under the brand name Adcirca and in generic form, are also approved; however, they must be swallowed whole. Tadliq provides an alternative for people with difficulty swallowing.
Tadalafil is also a component of the PAH combination therapy Opsynvi and is the main ingredient in Cialis, a medication for erectile dysfunction and noncancerous enlargements of the prostate gland.
Therapy snapshot
| Brand name | Tadliq |
| Chemical name | Tadalafil |
| Usage | Used to improve exercise ability in people with PAH |
| Administration | Liquid oral suspension |
Who can take Tadliq?
In the U.S., Tadliq is approved to improve exercise ability in people with PAH (World Health Organization Group 1). U.S. regulators have not established the therapy’s safety and effectiveness in pediatric patients.
Tadliq is contraindicated, or should not be used, by:
- individuals taking organic nitrates or guanylate cyclase stimulators such as the PAH therapy Adempas
- people with a history of known hypersensitivity, or severe immune reactions, to tadalafil, or the medications Tadliq, Adcirca, or Cialis
The FDA also recommends that Tadliq be used with caution in patients with certain other cardiovascular diseases. Although not specifically contraindicated, Tadliq is not recommended for people who:
- have pulmonary veno-occlusive disease> (PVOD), a rare cause of PAH
- have severe kidney or liver disease
- are currently using Cialis, Adcirca, or other PDE5 inhibitors
How is Tadliq administered?
Tadliq comes as a ready to use, peppermint-flavored liquid oral suspension that can be taken at home. The recommended dose is 40 mg (10 mL), taken once daily, with or without food.
This dosage may require adjustments for individuals using the antiviral medication ritonavir and for people with mild or moderate kidney or liver impairments.
Tadliq in clinical trials
The FDA’s approval of Tadliq was based on unpublished data from CMP demonstrating that this formulation is biologically equivalent to tadalafil oral tablets. The decision also took into account clinical trial data that demonstrated the effectiveness of the oral tablets — namely, Adcirca — in people with PAH.
The bioequivalence studies established that the two medications release the active ingredient into the bloodstream at the same rate and amount, and are thus expected to have similar clinical effects.
Efficacy data came from the Phase 3 PHIRST-1 trial (NCT00125918), which tested four doses of Adcirca against a placebo in PAH patients ages 12 and older. The data showed that, compared with the placebo, tadalafil was effective at the approved dose (40 mg). Treatment was found to:
- increase exercise abilities after four months, measured using a test to evaluate the distance that can be walked in six minutes
- significantly delay and reduce rates of clinical worsening
- improve measures of health-related quality of life
After PHIRST-1, participants could enter a long-term, open-label extension study called PHIRST-2 (NCT00549302). Data from that study showed that participants taking Adcirca maintained gains in exercise capacity after a year of treatment.
Tadliq side effects
The most common side effect associated with Tadliq is headache.
Less common, but potentially serious side effects may include:
- low blood pressure, especially for individuals with underlying cardiovascular diseases
- sudden vision loss
- sudden decrease or loss of hearing
- prolonged, painful erections
- a worsening of PVOD
If signs of fluid accumulation in the lungs occur with Tadliq, patients should be evaluated for PVOD. If that rare cause is confirmed, the use of Tadliq is not recommended.
Tadliq can interact with other medications and substances, including alcohol and PDE5 inhibitors. Patients should tell their healthcare providers about all medications they are using, and talk with them about safe alcohol consumption while using Tadliq.
Pulmonary Hypertension News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 


