Opsynvi (macitentan and tadalafil) for pulmonary hypertension
Last updated March 26, 2024, by Lindsey Shapiro, PhD

What is Opsynvi for pulmonary hypertension?
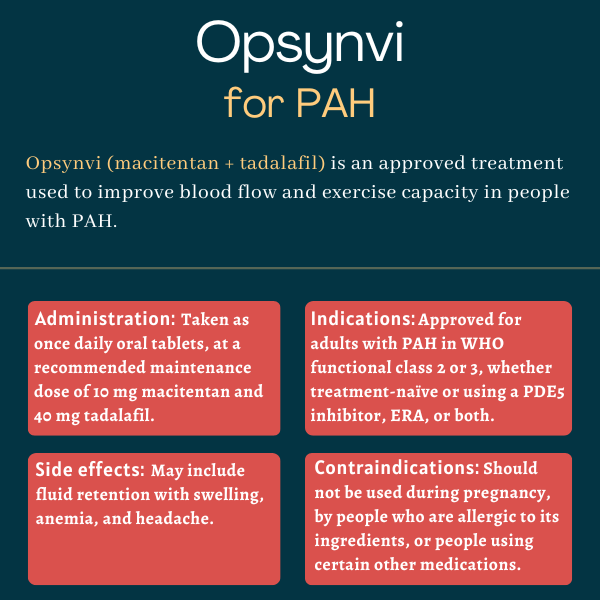
Opsynvi (macitentan and tadalafil) is an oral therapy approved in the U.S. for treating adults with pulmonary arterial hypertension (PAH) in World Health Organization functional class 2 or 3. It’s designed to reduce the risk of clinical worsening and hospitalizations, while improving exercise capacity in patients.
The medication contains a fixed-dose combination of macitentan and tadalafil, an endothelin receptor antagonist (ERA) and a phosphodiesterase 5 (PDE5) inhibitor that are sold separately as PAH treatments.
Marketed by Johnson & Johnson Innovative Medicine, Opsynvi is the first single-tablet combination treatment available to PAH patients in the U.S.
Therapy snapshot
| Brand name: | Opsynvi |
| Chemical name: | Macitentan and tadalafil |
| Usage: | Treatment for PAH patients in WHO functional class 2 or 3; can improve blood flow and exercise capacity |
| Administration: | Oral tablets |
How does Opsynvi work?
PAH is characterized by a narrowing of the vessels that carry blood from the heart to the lungs — called the pulmonary arteries — making blood flow through them more difficult, causing strain on the heart and making exercise more difficult.
Opsynvi is a fixed-dose combination of two molecules — macitentan and tadalafil — that each work via different mechanisms to cause the blood vessels to relax and widen, enabling better blood flow.
Blood vessel constriction (vasoconstriction) or relaxation (vasodilation) is mediated by the smooth muscles that line their walls. Macitentan works to block a signaling pathway that promotes vasoconstriction. It prevents the activation of endothelin receptor proteins that drive contractions of the smooth muscles. Individually, it is marketed as a PAH therapy called Opsumit.
Tadalafil works to boost a signaling pathway that promotes vasodilation. A molecule called cGMP normally helps relax the smooth muscles of the artery walls, and the PDE5 enzyme works to break down cGMP. Tadalafil inhibits PDE5 in order to increase cGMP levels and relax blood vessels. It first became available for PAH under the brand name Adcirca.
According to Opsynvi’s prescribing label, macitentan alone works to reduce the risk of clinical worsening and hospitalization, while tadalafil improves exercise abilities in PAH patients. ERAs and PDE5 inhibitors are recommended to be used together as a first-line treatment for many PAH patients. Prior to Opsynvi, which combines these medications in a single tablet, they had to be taken separately.
Who can use Opsynvi?
Opsynvi was approved by the U.S. Food and Drug Administration in March 2024 for the treatment of adults with PAH in WHO functional class 2 or 3. This action marked the first approval of a single-tablet combination therapy for PAH in the U.S.
The medication can be used by patients new to a disease treatment, as well as by those already taking a PDE5 inhibitor, an ERA, or both.
Opsynvi also is approved in Canada and in Argentina as a more convenient treatment for PAH patients already on a stable dose of a PDE5 inhibitor and an ERA separately.
Who should not use Opsynvi?
Opsynvi may cause harm to a developing fetus (a teratogenic medication), and it comes with a boxed warning that it should not be used in pregnant patients. Among women of reproductive age, pregnancy must be ruled out before starting treatment, monthly during treatment, and one month after stopping Opsynvi. Acceptable methods of contraception should be used while on the treatment.
Opsynvi is available to female patients only through a restricted access program called the Macitentan-Containing Products Risk Evaluation and Mitigation Strategy (REMS). This program ensures that only certified professionals can prescribe and dispense the medication to women, and that these patients are informed of potential risks to a developing fetus and take appropriate steps to prevent them.
Other contraindications have been noted for Opsynvi’s use:
- The medication is not recommended for patients with a history of exaggerated immune responses to macitentan, tadalafil, or any other ingredients in Opsynvi.
- It should not be given for simultaneous use with organic nitrates, either regularly or intermittently. Further, these medications should not be used until at least 48 hours after the last dose of Opsynvi.
- The treatment is not recommended for simultaneous use with guanylate cyclase stimulators such as riociguat, which also is approved to treat PAH.
Opsynvi also is not recommended for use by patients with significant kidney or liver impairments. Likewise, simultaneous use of Opsynvi with other medications that inhibit the CYP3A4 or CYP2C9 enzymes should be avoided.
How is Opsynvi administered?
Opsynvi is available as a fixed-dose oral tablet that’s taken once daily with or without food.
The film-coated oblong tablets come in two strengths:
- Pink tablets, containing 10 mg macitentan and 20 mg tadalafil, are labeled with “1020” on one side and “MT” on the other.
- White to off-white tablets, consisting of 10 mg macitentan and 40 mg tadalafil, are labeled with “1040” on one side and “MT” on the other.
The recommended maintenance dose for all patients is a single 10mg/40mg tablet once per day. Patients transitioning from PDE5 inhibitor monotherapy or a combination of PDE5 inhibitor and an ERA can start treatment at this dose.
For patients who have not previously been treated or are transitioning from ERA monotherapy, it is recommended they start with a lower daily dose (10mg/20mg) for one week. If this dose is well tolerated, patients can titrate up to the maintenance dose.
Tablets should be swallowed whole with water and not cut, crushed, or chewed. If a dose is missed, the tablet should be taken as soon as possible, and the next dose taken at the regularly scheduled time. Patients should not take two doses at the same time if a dose is missed.
Opsynvi in clinical trials
The approval of Opsynvi was largely backed by data from the pivotal Phase 3 A DUE clinical trial (NCT03904693), which enrolled 187 adult PAH patients in WHO functional class 2 or 3, who either had not previously been treated or had been on stable PDE5i or ERA monotherapy. Participants were assigned to receive Opsynvi at its approved maintenance dose, or to macitentan alone (10 mg) or tadalafil alone (40 mg) daily for about four months.
Published trial results showed the combination therapy led to significantly greater improvements in blood flow than either monotherapy alone. Specifically, Opsynvi was associated with a significant 28%-29% reduction in pulmonary vascular resistance — a measure of the resistance that must be overcome for blood to flow — relative to either monotherapy. Those benefits were observed regardless of patients’ prior treatment history, age, sex, race, or WHO functional class.
Treatment with Opsynvi also led to clinically meaningful, but not statistically significant, improvements in exercise capacity relative to those on macitentan or tadalafil. All three groups saw significant decreases in NT-proBNP, a biomarker of heart failure, with significantly greater decreases observed in the Opsynvi group. Patients in all three groups reported a similar easing of cardiopulmonary and cardiovascular symptoms, and few people experienced a WHO functional class worsening during the trial.
After the main trial, patients were able to enter an open-label treatment period, in which all are receiving Opsynvi for up to two years.
Common side effects of Opsynvi
The most common side effects associated with Opsynvi include:
- swelling caused by fluid retention
- anemia, or a lack of healthy red blood cells or of hemoglobin to carry oxygen to the body’s tissues
- headache or migraine.
Pregnancy and breastfeeding
Macitentan is consistently found to have teratogenic effects in preclinical studies, and for this reason, Opsynvi comes with a boxed warning that it should not be used during pregnancy due to the risk of birth defects. Negative pregnancy tests and effective contraception throughout treatment are required. If pregnancy is suspected while using Opsynvi, a patient should immediately contact their doctor.
Due to this risk, the Macitentan-Containing Products REMS requires that physicians or pharmacists prescribing or dispensing Opsynvi are certified and trained, and that all female patients are enrolled and compliant with pregnancy testing and contraception requirements.
Women on Opsynvi also are advised not to breastfeed while using the medication.
Liver toxicity
Opsynvi may cause liver problems, so liver enzyme tests should be obtained prior to starting Opsynvi and repeated during treatment as needed. Opsynvi should not be started in patients with significant liver enzyme elevations.
Patients should report symptoms indicative of liver injury, such as nausea, vomiting, upper right abdominal pain, fatigue, loss of appetite, yellowing skin, dark urine, fever, or itching while on treatment. If signs of liver injury become evident, Opsynvi should be stopped, but it may be started again in patients who see a normalization in liver enzymes.
Low blood pressure
Opsynvi may lead to temporary decreases in blood pressure (hypotension), and its use should be carefully considered in patients with underlying cardiovascular disease, existing hypotension, or who have other risk factors.
Certain other substances, such as alpha-1 blockers and antihypertensive medications also may have blood pressure-lowering effects when used with Opsynvi. Patients should talk with their doctors about any medications they will be using alongside Opsynvi.
Opsynvi combined with high amounts of alcohol can decrease blood pressure, increase heart rate, and cause dizziness or headache. Patients are advised not to have more than four alcohol-containing drinks in a short period of time while on Opsynvi.
Anemia
Opsynvi may cause early decreases in hemoglobin — the protein that enables red blood cells to carry oxygen through the body — reflecting anemia, which usually stabilizes over time. Hemoglobin should be measured before and during treatment as needed; starting Opsynvi in patients with severe anemia is not recommended.
Worsening pulmonary veno-occlusive disease
Vasodilators may worsen the cardiovascular status of people with pulmonary veno-occlusive disease (PVOD), a rare PAH subtype characterized by the narrowing of veins. Opsynvi is not recommended for PVOD patients. If signs of fluid buildup in the lungs are observed when Opsynvi is administered, PVOD should be suspected and Opsynvi stopped if confirmed.
Vision loss and hearing impairment
An eye condition leading to vision loss called non-arteritic anterior ischemic optic neuropathy (NAION) has been associated with tadalafil, with most affected patients having existing risk factors. Opsynvi is not recommended for people with known hereditary degenerative retinal disorders.
Tadalafil also has been linked to cases of sudden hearing loss, along with ringing in the ears and dizziness, but it is not known whether these symptoms are directly related to the medication. Patients should tell their doctor if they have these symptoms.
Fluid retention
Fluid retention and swelling are known side effects of ERAs, but they are also a clinical consequence of PAH. Heart failure has occurred in patients using Opsynvi, and patients with underlying left heart disease may be at particular risk. Patients should be monitored for signs of fluid retention. If clinically significant retention occurs, patients should be evaluated to determine the cause and the possible need to stop Opsynvi.
Decreased sperm count and prolonged erection
Macitentan and other ERAs can have an adverse effect on sperm counts, and male patients should be advised about their potential impact on fertility.
PDE5 inhibitors such as tadalafil also are used to treat erectile dysfunction and, as such, may be associated with prolonged or painful erections (priapism) that can damage the erectile tissue. Patients who experience an erection lasting longer than four hours, whether painful or not, should seek emergency care. The safety of using Opsynvi with other PDE5 inhibitors or other erectile dysfunction medications is not established, and other PDE5 inhibitors should be avoided when taking Opsynvi.
Pulmonary Hypertension News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Heart and lung machine boosts survival for pregnant women with PAH
- Plant-based echinacoside shown to ease signs of PAH in rat study
- How to explain the complexities of pulmonary hypertension to others
- Experts create new tool to speed pulmonary hypertension diagnosis
- Cold fronts and PH don’t mix, but there’s ways to combat winter pain
Related articles





