Tyvaso (inhaled treprostinil) for pulmonary hypertension
What is Tyvaso for pulmonary hypertension?
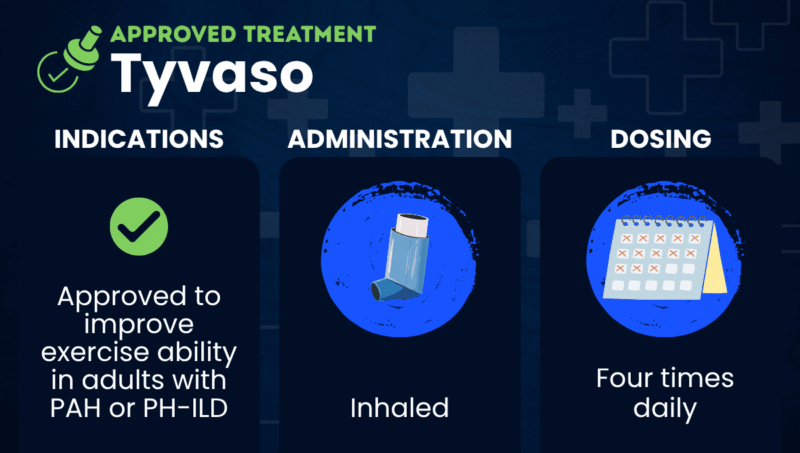
Tyvaso (treprostinil) is an inhaled therapy that’s approved to improve exercise ability in adults with pulmonary arterial hypertension (PAH) or pulmonary hypertension associated with interstitial lung disease (PH-ILD).
PAH and PH-ILD are forms of pulmonary hypertension, which is characterized by elevated pressure in the pulmonary arteries that carry blood from the heart to the lungs. This impedes blood flow and reduces the amount of oxygen in circulation, making physical activity more difficult.
The active ingredient in Tyvaso is treprostinil, a lab-made version of prostacyclin, a naturally occurring molecule that causes blood vessels to relax and widen. When inhaled directly into the lungs, it helps reduce pressure and improve blood flow through the pulmonary arteries to ease disease symptoms.
Tyvaso is a liquid solution that’s inhaled using a nebulizer. The medication also comes as a dry powder that’s inhaled through a portable inhalation device, which is called Tyvaso DPI. Both are marketed by United Therapeutics.
United also markets two other formulations of treprostinil for people with PAH. One, Remodulin, is given by infusion, while the other, Orenitram, is taken orally.
Therapy snapshot
| Brand name | Tyvaso or Tyvaso DPI |
| Chemical name | Treprostinil |
| Usage | Used to improve exercise ability in adults with PAH or PH-ILD |
| Administration | Inhaled into the lungs via nebulizer or portable inhalation device |
Who can take Tyvaso?
Tyvaso and Tyvaso DPI are both approved in the U.S. to improve exercise ability in adults with PAH and PH-ILD, corresponding to Groups 1 and 3 of the World Health Organization’s classification system.
There are no contraindications for the use of Tyvaso or Tyvaso DPI.
How is Tyvaso administered?
Both Tyvaso and Tyvaso DPI are inhaled directly into the lungs four times per day. These sessions are typically spaced about four hours apart during waking hours.
Tyvaso comes as a liquid. It is placed into a portable nebulizer that turns the medication into an inhalable mist. A single breath delivers about 6 micrograms (mcg) of treprostinil.
- The initial recommended dosage is three breaths (18 mcg) per treatment session. This will be slowly increased to a target maintenance dose of 9-12 breaths per session, as tolerated, and reduced if needed. Each session takes about 2-3 minutes.
Tyvaso DPI comes as a dry powder in single-dose cartridges, which are administered with a small, portable inhalation device.
- The initial recommended dosage is one 16 mcg cartridge per treatment session. This will be slowly increased to a target maintenance dose of 48 to 64 mcg per session, as tolerated. For most patients, each dose is taken in a single breath lasting less than two seconds.
Patients will be instructed on how to clean and care for the specific inhalation device that they are using.
Tyvaso in clinical trials
Tyvaso’s approvals for PAH and PH-ILD were supported by data from two Phase 3 clinical trials.
- The TRIUMPH study (NCT00147199) enrolled 235 adults with PAH who were already being treated with Tracleer (bosentan) or Revatio (sildenafil). The data showed that Tyvaso was associated with significant improvements in exercise capacity compared with a placebo after about three months. The clinical benefits were sustained long-term.
- The INCREASE trial (NCT02630316) involved 326 adults with PH-ILD. Its data showed that Tyvaso led to significant improvements in exercise ability compared with a placebo after about four months. The treatment was also associated with reductions in NT-proBNP, a biomarker of heart failure, and lower rates of clinical worsening relative to the placebo.
The approval of Tyvaso DPI was based on data from an open-label Phase 1 clinical trial called BREEZE (NCT03950739). In this study, 51 adults with PAH who were on Tyvaso switched to Tyvaso DPI. In results showed that:
- the switch to Tyvaso DPI was safe and well tolerated, with improvements in patient satisfaction
- Tyvaso DPI was associated with improvements in exercise capacity after three weeks, which were sustained long term
Tyvaso side effects
The most common side effects reported with Tyvaso and Tyvaso DPI were:
- cough
- headache
- nausea
- throat irritation and pain
- fainting
- flushing, or a reddening of the face and neck
Dizziness and diarrhea were also seen with Tyvaso, while shortness of breath was experienced with Tyvaso DPI.
Both Tyvaso and Tyvaso DPI can cause serious adverse events, including:
- symptomatic hypotension, or low blood pressure causing noticeable symptoms
- increased risk of bleeding
- bronchospasms, a tightening of the muscles in the airways, especially for people with a history of certain airway diseases
Certain medications may affect the safety or efficacy of Tyvaso. Patients should inform their healthcare provider about all other medications they are taking.
Pulmonary Hypertension News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.

 Fact-checked by
Fact-checked by